CCS 新鮮胚胎植入的 "7大" 理由,根據RMANJ紐澤西團隊斯考特Scott的論述,在生育與節育期刊提出 7大鐵證 ,證明進行CCS不用冷凍胚胎,新鮮胚胎植入就大可進行,理由有:
(1)因為CCS的正確性和有效性可以大幅提高成功率和胚胎著床率。
(2)提高試管嬰兒成功率是最大的考慮,而胚胎染色體異常比率從25%增加到40歲之後的85%,這使得更需要做CCS。
(3)因為CCS的費用實在是很昂貴,也因此能夠讓病人少錢就是一個patient center care,因此是不是能夠減少病人冷凍胚胎的費用呢?
(4)使用CCS可以減少多胞胎的比率,因為染色體正常就可以進行單一胚胎植入,進行BEST也就是所謂的囊胚期染色體正常單一胚胎植入。根據研究單胞胎和雙胞胎小孩子的體重,單胞胎會比雙胞胎增加650公克。
(5)冷凍胚胎其實是不可以不用的,因為有CCS之後會有單一胞胎植入,單一胚胎植入之後會有冷凍胚胎,這個冷凍胚胎的對象是染色體正常的胚胎。
(6)有一些病人並不能從CCS得到好處,只有部分的病人,比如根據RCT三份的研究發現,如果說打針病人年紀大,取卵數少,AMH低,或者是形成囊胚期胚胎率低,這個做CCS並沒有得到好處。
(7)能夠進行CCS一定要:
1.進行囊胚期胚胎培養、
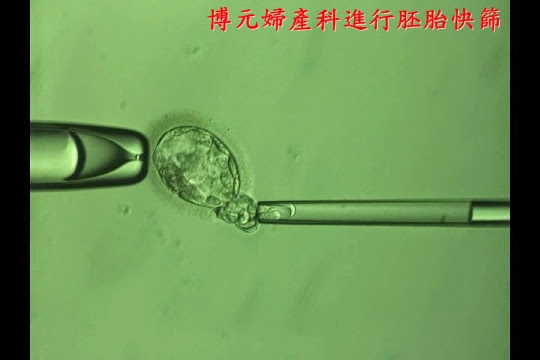
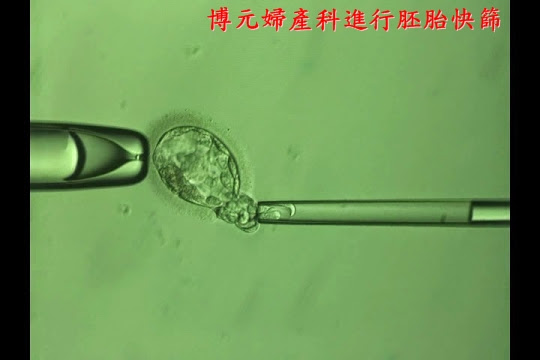
2.能夠進行胚胎切片、
3.能夠進行快速冷凍的方法。
因此這三大要件都存在的實驗室,才能夠進行CCS新鮮胚胎植入,並不是每一家試管嬰兒中心都有這3個技術這個能力,因此他提出因為有這個7大理由的時機已經成熟,
也因此
進行胚胎全基因放大檢測胚胎的染色體,
進行胚胎的植入"新鮮"胚胎植入,妳不必再冷凍胚胎苦苦等報告了,這一份的學士論文是登在生育與節育期刊2014年9月份。
參考:
Comprehensive chromosome screening with
synchronous blastocyst transfer: time for a
paradigm shift*
Recently, the nature of assisted reproductive technology
(ART) laboratory investigation has been shifting. Tradition-
ally, it has focused on optimizing the culture milieu or assur-
ing fertilization; now, a variety of new technologies are
available to assess the reproductive potential of individual
embryos. Perhaps most prominent has been the resurgence
of embryonic aneuploidy screening. The validation of
24-chromosome testing platforms has led to a variety of
studies demonstrating higher implantation and delivery rates.
These findings are now translating to changes in the para-
digm of ART practice.
Caution is prudent in times of change, and methodical
analyses are needed. Evaluation logically focuses on efficacy
in terms of enhanced implantation and delivery rates. Other
factors, such as safety, cost, and accessibility also deserve
thoughtful consideration. Evaluations of these endpoints
should take into account the caliber of the data supporting
the ‘‘new paradigm,’’ in parallel with the data supporting
the current ‘‘standard of care,’’ and both should be evaluated
with the same level of rigor.
Several investigators have recently expressed concerns
about the implementation of comprehensive chromosomal
screening (CCS) in clinical practice. Fortunately, an ever-
growing literature is available to provide clinicians and scien-
tists with the information they need to evaluate many of the
critical issues. Some of the major issues and questions
include:
1. Efficacy of 24-chromosome embryonic aneuploidy
screening. Multiple studies provide class I data demon-
strating higher implantation and delivery rates following
24-chromosome aneuploidy screening. In distinct contrast
to fluorescent in-situ hybridization-based preimplantation
genetic screening studies in which every randomized
controlled trial (RCT) showed either no improvement or
active detriment, every RCT involving 24-chromosome
screening has demonstrated benefit (1–3).
2. What magnitude of improvement in clinical outcomes is
necessary to justify screening? Answering this question
inevitably involves a subjective decision that will be
made by patients after counseling by the clinicians caring
for them. Given that aneuploidy rates vary from 25% in
women in their late twenties to 85% for those in their
mid-forties, the opportunity for enhancing outcomes will
be greatly affected by the age of the female partner and
her intrinsic ovarian responsiveness. It is unlikely that im-
provements will be made in direct proportion to the aneu-
ploidy rate, as many other factors affect delivery rates.
Women with high embryonic arrest rates are unlikely to
attain the full benefit of screening. Still, the magnitude of
the enhanced outcomes seen in the RCTs is substantial.
3. The cost of CCS may be burdensome. Although substantial
costs are associated with CCS, even in proportion, they are
* This is an open access article under the CC BY-NC-ND license (http:// would appear that this type of screening is appropriate
660 VOL. 102 NO. 3 / SEPTEMBER 2014
lower than the costs of additional ART cycles. A definitive
cost-effectiveness study has not been published to date.
Although enhanced delivery rates should translate to fewer
treatment cycles, that question must await more detailed
analyses before conclusions may be drawn. Additionally,
savings attributable to decreased pregnancy losses and the
care provided to ongoing aneuploid gestations would need
to be considered. Given that, and the impact on transfer or-
der discussed below, it is unlikely that cost effectiveness will
limit implementation of embryonic aneuploidy screening.
4. Implementation of CCS may actually increase the risk for
multiple gestations unless transfer order is reduced. That
very fact has already been established in a randomized
controlled trial (2). In fact, it is a mathematical certainty.
As implantation rates increase, if there is no decrease in
transfer order, then multiple gestation rates will inevitably
rise. However, it is not reasonable to assume that transfer
order would remain the same. For the first time, there are
class I data demonstrating eSET after CCS is as effective
as double-embryo transfer of unscreened embryos (2).
All prior RCTs comparing elective single-embryo transfer
(eSET) versus double-embryo transfer found poorer per-
transfer outcomes with eSET. If CCS is used, that is no
longer true. Equivalent delivery rates are maintained while
virtually eliminating the risk of twins. The paradigm using
CCS and eSET produced an average gain in birth weight of
approximately 650 grams. No other single intervention in
obstetrics has produced such a dramatic enhancement in
birth weight, which is known to be highly correlated
with the health of the child. Of course, the transfer of
two screened embryos would further increase pregnancy
rates, but at the cost of quite elevated twin rates; thus, it
should be discouraged. Armed with these data, utilization
of eSET in our program has risen from less than 6% to
approximately 60% over a 4-year interval.
5. Embryo cryopreservation is essential to the application of
CCS. This is an excellent point, as it is true in many, but not
all, programs. Analyses can be completed in as little as
4 hours, and several programs now have testing labora-
tories within their facilities. However, that may not be
necessary. Data from RCTs demonstrate equivalent deliv-
ery rates following the transfer of fresh or vitrified CCS
screened blastocysts (2). Furthermore, data now demon-
strate meaningfully better obstetrical outcomes in concep-
tions following the transfer of cryopreserved embryos.
6. Some subpopulations may not benefit from aneuploidy
screening. The studies to date have focused on infertile
normal responders. No class I data address the impact of
CCS in women who are low responders or have recurrent
pregnancy loss. An RCT to determine the impact of CCS
in women at risk for low response to gonadotropin stimu-
lation has been registered (NCT01977144) and is currently
underway. Within the general ART population, individuals
who might typically be considered candidates for two-
embryo transfer should be offered CCS. Given that the

.jpg)
.jpg)
.jpg)
.jpg)