肥胖多囊性卵巢症候群試管嬰兒新療法 pco ultra-long agonist (ULA) protocol
2014.06.07
肥胖多囊性卵巢症候群試管嬰兒新療法 pco ultra-long agonist (ULA) protocol
我們常遇到多囊性卵巢症候群的試管嬰兒患者有過度肥胖的情形,
有一份來自於2014年《婦產科內分泌雜誌》的研究,
他們發現超長療程ULP懷孕率有70.2%,反觀LP長療程為5
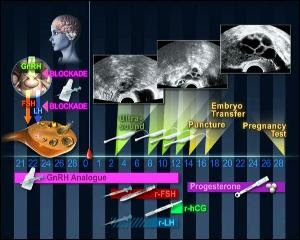
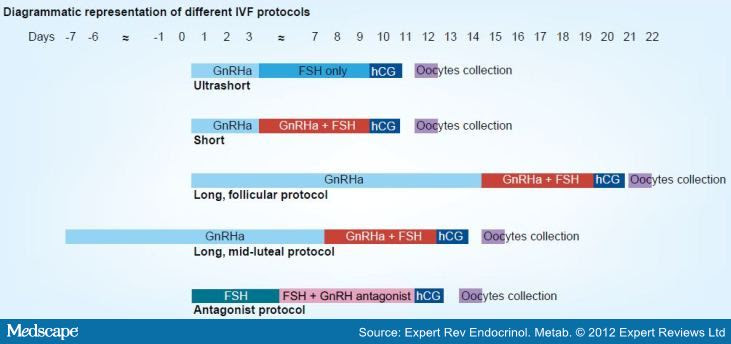%20protocol.jpg)
Gynecol Endocrinol. 2014 Mar;30(3):209-12. doi: 10.3109/09513590.2013.860121. Epub 2013 Dec 19.
A novel modified ultra-long agonist protocol improves the outcome of high body mass index women with polycystic ovary syndrome undergoingIVF/ICSI.
Abstract
In an attempt to evaluate the effectiveness of a novel modified ultra-long agonist (ULA) protocol on polycystic ovary syndrome (PCOS) patients undergoing in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI), a retrospective study of 499 women employed with either ULA or conventional long agonist (LA) protocol was analyzed. In high BMI group (>25 kg/m(2)), the ULA protocol yielded significant higher clinic pregnancy rate (PR) (70.2% versus 50.8%, p < 0.05), implantation rate (52.7% versus 35.7%, p < 0.05) and live birth rate (63.8% versus 39.0%, p < 0.05) when compared with LA protocol. In low BMI group (≤25 kg/m(2)), the ULA protocol also demonstrated a higher clinic PR (70.8% versus 59.5%, p < 0.05) whereas implantation rate and live birth rate are comparable. Within ULA protocol, the clinic PR, implantation rate and live birth rate are similar between high and low BMI patients. Similarly, the clinic PR and live birth rate demonstrated no significant difference within LA group but there is a significant lower implantation rate (35.7% versus 63.9%, p < 0.05) observed in high BMI patients. No difference in miscarriage rate and severe OHSS rate was found among all groups. In conclusion, ULA protocol benefits the IVFoutcomes of PCOS patients with high BMI status.






